Zydis® Fast Dissolve Technology Platform
 The Zydis® ODT (orally dissolving tablet) fast-dissolve formulation, is a unique, freeze-dried oral solid dosage form that disperses almost instantly in the mouth – no water required. With more than 20 products launched in 60 countries, it continues to be the world’s best-in-class ODT technology. Our Zydis® fast dissolve team is the best trained in the world, offering feasibility evaluations as well as support across the entire lifecycle of your product. Whether you are considering an ODT to enhance pharmacokinetics through pre-gastric absorption, looking for a way to improve patient compliance, or seeking a marketing advantage for a valued brand, Zydis® fast dissolve can help enhance the value of your investment and accelerate your product’s potential.
The Zydis® ODT (orally dissolving tablet) fast-dissolve formulation, is a unique, freeze-dried oral solid dosage form that disperses almost instantly in the mouth – no water required. With more than 20 products launched in 60 countries, it continues to be the world’s best-in-class ODT technology. Our Zydis® fast dissolve team is the best trained in the world, offering feasibility evaluations as well as support across the entire lifecycle of your product. Whether you are considering an ODT to enhance pharmacokinetics through pre-gastric absorption, looking for a way to improve patient compliance, or seeking a marketing advantage for a valued brand, Zydis® fast dissolve can help enhance the value of your investment and accelerate your product’s potential.CUSTOMER BENEFITS
- Better pregastric absorption for certain drug compounds
- More efficient delivery
- Ideal for certain indications
- Protection from counterfeiting
- A wider range of application:
- Dysphagia
- Pediatric and geriatric application
- Fast Onset
- Ease of use
- A wide range of therapeutic areas
- Anti-psychotic (Parkinson’s disease, schizophrenia)
- Anti-emetic (Travel sickness)
- Gastro (Diarrhea, Constipation)
- Allergy (Anti histamine, immunotherapy)
- Anxiolytic (Anti-depressants)
Case Study: Zydis® Fast Dissolve Technologies – Rapid Onset in the Treatment of Migraine
VALUABLE PRODUCT DIFFERENTIATION
- High level of customer preference
- Enhanced market appeal
- Rapid onset of action
- Multiple colors and shapes
- Embossing with corporate logos and product codes
- Unique packaging, including child-resistant options
- Taste masking and flavors formulated for specific markets, including pediatrics and veterinary medicine
HOW IT WORKS
ZYDIS® ULTRA
- Same dissolution profile
- Greater taste masking capabilities
- Increased doses > 400mg
- Functional coating for controlled / sustained release applications
- High speed manufacturing
- On-line printing for flexible solutions
- Wider range of application
Ideal for consumer health (OTC), and to mask bitter or strong tasting API
ZYDIS® BIO
The challenges of oral delivery of proteins & peptides well documented and are not limited to:
- pH
- Peptidase & proteolytic enzyme activity
- Potential interaction with other constituents of GI fluids
- High molecular weight
Zydis® Bio sub-lingual delivery overcomes these challenges:
- Avoids harsh environment of GIT
- Use of bio-adhesives / absorption enhancers
- Pre-gastric delivery (e.g. buccal, sublingual, oesophageal)
- pH relatively neutral (5.5 – 7.2 depending on salivary flow rate)
Benefits of Zydis® Bio for peptide & protein drugs
- Potential for sublingual / buccal absorption
- Solid, unit doses presented in protective pack
- Low temperature processing minimizes manufacturing losses of labile drugs
- Solution / suspension dosing achieves good content uniformity for low dose actives
- Solid dosage form and low water activity aids long term stability
- Liquid processing facilitates containment of potent drugs in production
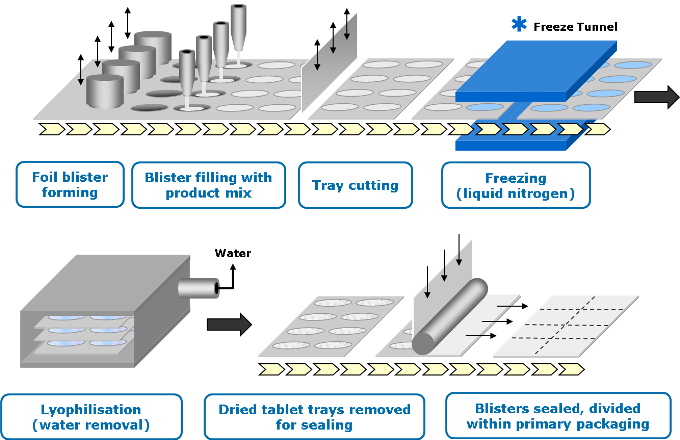
HOW IT IS MADE
Stage 1 – Mixing
The bulk API is formulated into a liquid solution or suspension.
Stage 2 – Filling and freezing
The liquid is precisely filled into pre-formed blisters and passed through a specially designed cryogenic freezing process to control the ultimate size of the ice crystals.
Stage 3 – Lyophilization
The frozen units are then transferred to large-scale freeze dryers for the lyophilization process.
Stage 4 – Sealing
The blisters containing the dried Zydis® units are then sealed via a heat-seal process to protect the product from varying environmental conditions and ensure long-term stability.
CATALENT CAPABILITIES
- Feasibility programs & development teams
- Tailored to meet the known product requirements such as API unit dose
- Consideration of the relevant API characteristics identified during the technical evaluation of preformulation data
- A range of prototype Zydis® formulations prepared under different processing conditions (bench-scale); analytical techniques applied as appropriate to determine the compatibility of a candidate API with the Zydis® technology
- Short-term (4 week) accelerated physical stability studies typically undertaken before recommendations for a full development program are made
- Operational excellence and efficiency via Lean Six Sigma principles
- Full in-house analytical and regulatory services
- Full characterization of your API and associated Zydis® formulations via expert analysis and interpretation of data throughout the development process to provide a robust data package in support of regulatory filings
- Smart full-lifecycle management from molecule to market – with Lean efficiency standards
- Expert handling and maximization of potent and controlled drugs
- Bench, pilot, and full-scale cGMP manufacturing
CATALENT SERVICES
Optical microscopy
Characterizes the API crystal form and identifies any changes during formulation and processing
Freeze-drying microscopy
Identifies critical formulation parameters such as collapse temperature, eutectic/glass transition temperature
Particle size analysis (laser diffraction)
Determines particle size ranges relevant to Zydis® suspension stability for insoluable API
Dynamic Vapor Sorption
Characterizes moisture sorption properties and physical stability of lyophilized formulation in humidity
Characterization of hydrates and polymorphs
X-ray powder diffraction (XRPD)
Characterizes solid-state phrase behavior
Characterization of moisture sorption properties and physical analytical chemistry (HPLC, UV, NIR, FTIR)
Chemical analysis of API in support of preformulation studies (e.g., solubility determinations, compatibility testing)
FACILITIES
- Swindon, U.K.
- Research and development
- Pilot line with controlled and potent drug capabilities
- FDA and MCA audited Controlled drug capabilities
All of our sites are subject to annual review so we can remain in highest compliance with:
- FDA/cGMP
- Canadian HPB
- British MHRA
- European Community


