OptiMelt® Hot Melt Extrusion
ENHANCE BIOAVAILABILITY OF YOUR POORLY SOLUBLE API WHICH WILL LEAD TO BETTER TREATMENTS FOR YOUR PATIENTS
OptiMelt® is Catalent’s unique ability to formulate, develop and commercialize hot melt extrusion processes and integrate these into differentiated final dosage forms.
The main drivers of increased hot melt extrusion use in the pharmaceutical and OTC industry are:
- Enhances solubility/bioavailability for poorly soluble drugs
- Continuous processing allows for good process control & scalability
- Solvent-free (unlike some alternative solid dispersion approaches)
- Extrudate is versatile in its end use, including potential incorporation in controlled release delivery formulations
- Ability to incorporate taste masking
Hot melt extrusion is typically co-located with downstream processing and solid dose form manufacturing.
OptiMelt® broadly addresses bioavailability enhancement factors at any stage of drug development through commercialization, with integrated teams of scientists and technologies providing the following value:
- Increase development success rate of poorly soluble drugs
- Optimize product efficacy, safety, and release properties
- Reduce time to market
OPTIMELT® CAPABILITIES
Catalent’s OptiMelt® platform of integrated bioavailability enhancement technologies provide a holistic approach to optimizing poorly soluble drugs and development programs.
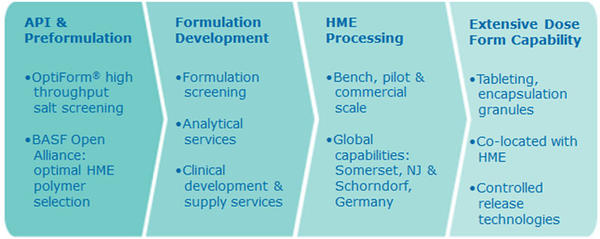
- Pilot to Development to Commercial scale hot melt extrusion, with global capabilities
- Broadest selection of downstream processing technologies, co-located with OptiMelt™ hot melt extrusion at all OptiMelt™ facilities
- Integrated solutions provider, with over 75 years of industry experience
- Formulation acceleration and optimization with open Catalent-BASF bioavailability alliance
HOW IT WORKS
OptiMelt® hot melt extrusion enhances solubility by producing a stable, amorphous solid dispersion, increased-energy form through a combination of the process and the chemical properties of the excipient. The resulting product, called the extrudate, is then further processed and converted into a final dosage form to achieve the final drug delivery profile desired.
CATALENT SERVICES
Through expert analysis and interpretation of data, our OptiMelt hot melt extrusion team will fully characterize your API and associated formulations throughout feasibility, development, and commercialization to provide a robust data package in support of regulatory filings. We will accelerate scale-up and technical transfer of your program at all product stages.
- Feasibility and Development programs
- Tailored to meet your product requirements
- Consideration of the relevant API characteristics identified during the technical evaluation of preformulation data
- A range of prototype formulations prepared under different processing conditions (bench-scale); analytical techniques applied as appropriate to determine optimum formulation and process parameters
- Short-term accelerated physical stability studies typically undertaken before recommendations for a full development program are made
- Full in-house analytical and regulatory services
- Full characterization of your API and associated formulations via expert analysis and interpretation of data throughout the development and scale-up process to provide a robust data package in support of regulatory filings
- Full-lifecycle management from molecule to market – with Lean efficiency standards
CATALENT OPTIMELT® FACILITIES
We currently have three OptiMelt hot melt extrusion facilities.
The facilities have co-located downstream processing capabilities to develop the hot melt extrusion extrudate into your desired final dosage form and controlled release functionality of your drug.
Across our OptiMelt facilities, our capabilities include:
- Potent handling
- DEA schedule drug handling
- Pilot feasibility scale extruders
- cGMP development and commercial scale extruders