
Catalent Xpress Pharmaceutics®
Integrated Development Offering to Facilitate Adaptive Trials and Accelerate Phase 1
Phase 1 clinical studies have traditionally been impeded by the reliance on two or more fixed dose strengths, and an inability for the clinic to dynamically adjust dosing based on clinical feedback. A significant opportunity exists therefore to optimize the traditional vertical development approach by increasing flexibility in dose selection.
Introducing Catalent Xpress Pharmaceutics®, a more advanced development offering that integrates formulation development with clinical manufacturing, regulatory support, and clinical testing, to help achieve flexible and efficient First-in-Human studies and fast development. At the center of this program is the ability to adjust both formulation and dose based on real-time clinical data, using on-demand manufacturing approaches and adaptive clinical design protocols. This offering helps optimize and accelerate the overall development process by reducing stability requirements, which can lead to significant reductions in costs, time, and the amount of API necessary.

4 to 6 Months vs 9 to 12 Months (Traditional CMC Model)
- Quick to First-in-Human and proof of concept studies using on-demand product manufacturing close to the point of administration
- Utilizes flexible formulation strategies for rapid dose adjustment at the phase 1 CRO
- Significant savings on both API and time
- Allows better decision making using real time clinical data
- Integrated approach that supports customers’ regulatory filing strategies.
HOW DOES CATALENT XPRESS PHARMACEUTICS WORK?
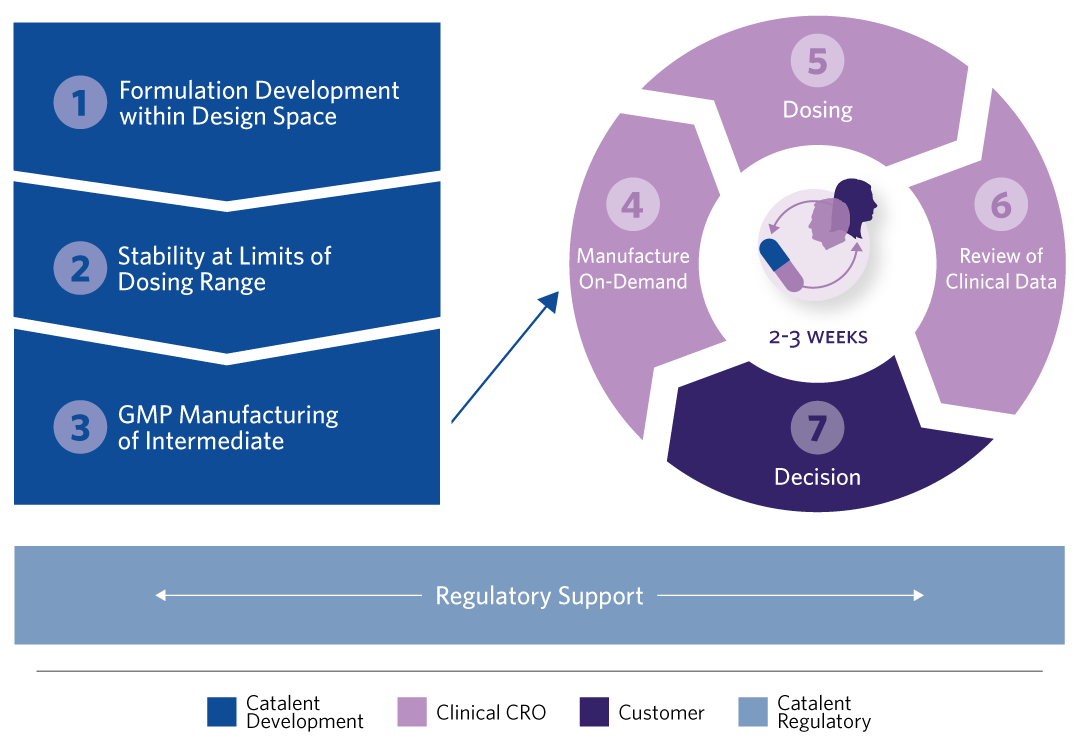
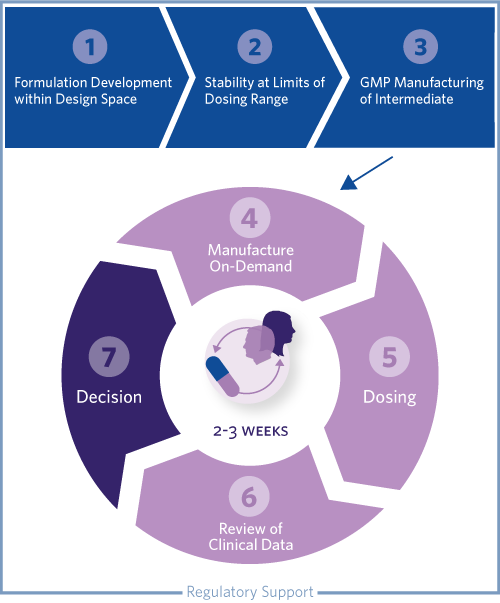
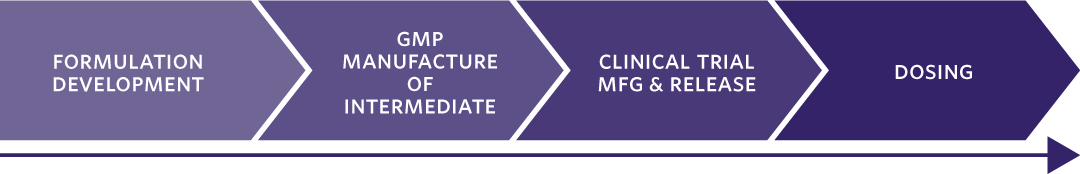
1. FORMULATION DEVELOPMENT Phase-appropriate, intermediate products are designed using a wide range of technologies (API/blend in bottle), for both simple or bio-enhanced (lipid-based); Mapping of the ‘formulation design space’ enables flexible choice of formulation, the dose, or both.
2. STABILITY OF GENERATED PRODUCTS AT THE LIMITS OF DOSING RANGE Sufficient ‘in-use’ stability is established in the intended presentation format at the limits of the dose range to be evaluated.
3. GMP MANUFACTURE OF INTERMEDIATE Flexible dosing by design. Single batches of intermediate formulations are manufactured and supplied for clinical testing.
4. ‘ON-DEMAND’ MANUFACTURING AT THE CLINICAL SITE The intermediate product is filled on-demand into capsules or reconstituted in bottles by the contract research organization’s (CROs) Phase 1 Clinic.
5 – 7. ITERATIVE DOSING, REVIEW OF CLINICAL DATA, AND DECISION-MAKING Dose increments are established based on emerging clinical data; Iterative cycles of up to three weeks between cohort dosing.
8. REGULATORY SUPPORT Focus on speed-to-clinic by identifying data that can be generated on non- clinical batches; Preparation of CMC section of regulatory submission with inclusion of justification for use of dose ranges, to allow rapid changes to dose during the clinical trial, alleviating the need for repetitive interactions with the regulatory authorities.
WHY PARTNER WITH CATALENT?
Catalent specializes in comprehensive development, analytical and bioavailability enhancement, and has a proven track record of optimizing thousands of molecules. With our rigorous data-driven scientific approach, deep development expertise, robust regulatory support and integrated collaboration with clinical research organization (CRO), we can help accelerate your molecule through phase 1. We have a dedicated scientific team to guide your molecule through the best development pathway, and offer customized solutions from pre-clinical through to clinical scale-up and launch to fit your needs.
AFTER YOUR CATALENT XPRESS PHARMACEUTICS PROGRAM
INTEGRATED SOLUTIONS
Xpress Pharmaceutics builds on Catalent’s extensive expertise and integrated offerings in accelerating the advancement of promising compounds into clinical and beyond including, OptiForm® Solution Suite and OptiForm™ Total Supply .
Achieve fast, flexible and efficient Phase 1 with Catalent Xpress Pharmaceutics®!
Catalent Xpress Pharmaceutics is a registered trademark of Catalent, Inc. or its affiliates or subsidiaries
